As a data analyst, your role is critical in producing reliable data from cross trial analysis. However, the clinical trial data you need is often scattered across multiple SharePoint sites – each created individually for separate trials. When you attempt cross-trial data analysis, you quickly encounter SharePoint’s limitations, as it simply isn’t built for querying data across multiple sites. SharePoint’s fragmented structure forces you to undergo manual, repetitive searches, costing you valuable time and risking accuracy.
Common SharePoint data challenges for pharma data analysts
Here’s what you might typically encounter managing your pharma data in SharePoint:
Fragmented data across multiple sites:
Your clinical trial data likely lives across numerous SharePoint sites, complicating rapid access. Fragmented data makes cross-trial reporting time-consuming and error-prone, leading to missed deadlines and frustrated teams.
Complex and slow reporting processes:
Creating comprehensive, accurate regulatory reports (e.g., FDA or EMA submissions) from scattered data is tedious and inefficient. Errors or inaccuracies can result in costly delays and compliance failures.
Limited querying and analytical capabilities:
You will no doubt face significant constraints due to SharePoint’s limited querying capabilities (of up to 5,000), making it challenging to perform complex cross-trial analysis or generate comprehensive insights. As a result, you’ll have to resort to manual exports, increasing the risk of data inconsistencies and reporting delays.
Heavy reliance on IT:
Depending on IT for data queries and complex reporting slows down your analysis and thus decision-making. For each IT request you have to make introduces delays, reducing your productivity and hindering your ability to drive agile, data-driven initiatives.
SharePoint’s built-in data limitations:
You’ll already be familiar with SharePoint inherent limitations, such as item query limits (up to 5000 items per view), complicating large-scale data analysis and management. While SharePoint has strong versioning and security, these built-in limitations still pose significant operational challenges for you.
AxioWorks SQList: Empowering pharma analytics with cross-trial data analysis
Whilst these challenges are common, it doesn’t have to be this way. Research from the American Data Network clearly shows that when pharma data is managed effectively it significantly reduces operational costs, streamlines workflows, and minimises medical errors – directly enhancing clinical trial outcomes.
But how can you manage your trial data in SharePoint more effectively?
Introducing SQList.
SQList solves these common yet critical challenges by effortlessly syncing SharePoint data with SQL Server, providing a centralised, always-current data source.
How SQList works
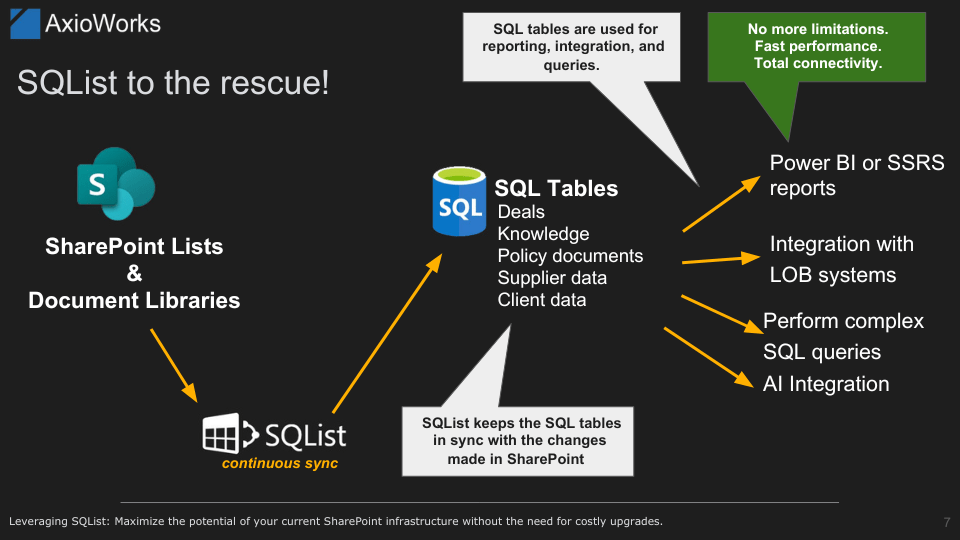
SQList acts as a bridge between your existing SharePoint environment and SQL Server, continuously exporting SharePoint lists and libraries as normalised SQL Server tables. It replicates data in near real-time, ensuring all updates made in SharePoint are immediately reflected within SQL Server without affecting your existing SharePoint structure or security settings.
Here’s how it works:
- Real-time replication: SQList automatically detects changes in SharePoint and updates the SQL Server database instantly. This near real-time data availability ensures you always have the most current data for accurate analytics and reporting.
- Simple setup and minimal disruption: SQList integrates easily with your existing SharePoint environment, requiring no complicated configurations or significant disruptions. It runs quietly in the background, maintaining data synchronisation seamlessly.
- Preserving data integrity: Data flows securely in one direction – from SharePoint to SQL Server – preventing accidental or unauthorised data modifications. Comprehensive audit logs further simplify regulatory compliance.
- Leveraging existing skills and tools: By making SharePoint data available in SQL Server, SQList lets your team leverage familiar and powerful reporting tools such as Power BI, SSRS, and other analytics platforms, without additional training or costly new software investments.
By efficiently bridging SharePoint’s data limitations, SQList enhances your ability to analyse and report on clinical trial data effortlessly.
Benefits of using SQList in pharma data management:
Centralised clinical trial data management:
SQList consolidates fragmented SharePoint data into organised, easily accessible SQL Server tables, significantly simplifying cross-trial data management and analysis.
Instant, cross-trial analytics in near real-time:
Continuous data updates allow immediate responses to compliance or operational issues, ensuring informed decisions based on current data.
Simplified reporting and advanced analytics:
Integrating effortlessly with Power BI, Tableau, Cognos, and SQL Server Reporting Services (SSRS) you can use SQList to produce accurate, timely, and comprehensive reports, addressing your needs for advanced analytical capabilities.
Reduced dependency on IT:
SQList empowers you and your team of data analysts to independently access, query, and analyse clinical data through familiar BI and reporting tools like Power BI, Tableau or Cognos or even direct SQL queries, reducing bottlenecks and significantly enhancing operational efficiency.
Assured data integrity and compliance:
Secure, one-way synchronisation from SharePoint to SQL Server significantly reduces the risk of data breaches or errors. Comprehensive audit logs simplify compliance efforts.
Real-world impact: SQList pharma analytics success story
A leading pharma firm faced significant challenges in data management, including slow reporting, limited analytical capabilities, and cumbersome cross-trial data retrieval from SharePoint. After implementing SQList, they experienced:
- Near real-time access to consolidated, cross-trial data.
- Dramatic improvements in analytics and reporting accuracy.
- Significant operational cost savings by leveraging existing BI tools and skills.
- Faster, more informed decision-making positively impacting trial outcomes.
One project manager noted: “Implementing SQList transformed our clinical trial reporting. We’ve transitioned from reactive scrambling to proactive management of compliance and timelines.”
Transform your pharma analytics with SQList
By introducing SQList, you can simplify your daily tasks:
- Quickly generate comprehensive, compliant reports.
- Perform seamless cross-trial data analysis in real-time.
- Improve collaboration with accurate, easily accessible data.
- Conduct audits confidently, knowing your data integrity is assured.
Embrace pharma’s digital transformation
Digital transformation in pharma is essential, not optional. Pharma companies adopting innovative solutions like SQList by AxioWorks position themselves for success, ensuring compliance, efficiency, and effectiveness in a rapidly evolving industry.
Ready to simplify your clinical trial data management and analytics?
Contact us today for a personalised SQList demo. Our experts are ready to help streamline your clinical trial analytics and achieve digital transformation success.